Synthego: The One Stop Shop for Revolutionary Genetic Research
This article is part of a series about how OS Fund (OSF) companies are radically redefining our future by rewriting the operating systems of life. Or as we prefer to think about it: Step 1: Put a dent into the universe. And Step 2: Rewrite the universe. You can see the full OSF collection here and read more about Building a Biological Immune System.
In contemplating the future, I love imagining how our daily lives today will be thought of in the future. What appears sci-fi to us today but will be “normal” 50 years from now? What inefficient and boneheaded things do we do today that future generations will look back and laugh at?
Seeing beyond what’s possible is a rare skill. Being able to design and build beyond what’s possible is even rarer. Put together, this is the unique set of skills and abilities that OSF founders all have in common. Most importantly, they’ve chosen to focus their abilities to tackle the biggest problems humanity faces.
But who are they? What makes them tick? Why do this versus other things? And how might their technologies change the world? These are their stories.
—
Michael and Paul Dabrowski run on the fuel of ambitious endeavors aimed at changing the course of humanity.
After leaving engineering jobs at SpaceX, they co-founded Synthego with a mission to improve how scientific research was performed by applying engineering-first principles and design thinking to biotechnology.
At Synthego, Michael and Paul have built a suite of genome editing tools and products based upon CRISPR that allow any researcher to access state of the art science, at scale — no matter if they’re working on basic science research, uncovering critical disease targets, or testing novel cell or gene-based therapeutics.
Synthego’s innovation has revolutionary potential across a very wide range of fields. Researchers working to understand impacts and applications of gene editing for everything from human disease to plant fertilization can devote their time, talent, and resources on their scientific focus, leaving the mechanics of CRISPR gene editing to Synthego. Outsourcing CRISPR to Synthego creates economies of scale, opportunities for learnings across very large data sets, and improvements in the process over thousands of iterations, all while freeing scientists and labs from needing to be CRISPR experts in addition to their specific area of expertise.
Synthego is particularly interested in one potential outcome: making cell and gene therapies as accessible as vaccines — doing so would make life-saving treatments accessible for everyone who needs them.
Upending the Status Quo
If you are one of the 20,000 or so people in the US who have Hemophilia B, a genetic disorder caused by a missing or defective clotting protein (factor IX), hope might be on the horizon. You might be able to put transfusions in the rearview mirror; you might be able to enjoy life without scrupulously avoiding activities that could cause even the smallest injury. You might be able to access a treatment that cures your broken DNA.
Hemophilia B is a monogenic disease, meaning it’s caused by a mutation on only a single gene. A promising new genetic therapy in clinical trials inserts a healthy version of the clotting factor gene into liver DNA, causing the cells to start producing the missing protein.
The catch? Each treatment costs $1 million, making it well out of reach for most who need it. Other gene therapies are similarly priced: a treatment for a blindness-causing retinal disorder costs $850,000.
If nothing changes, it’s safe to assume that as gene therapies come online for any of the 7,000+ known monogenic diseases — including cystic fibrosis, polycystic kidney disease, and Tay Sachs — they will be similarly inaccessible. Since therapies for more complex genetic disorders will themselves likely be more complex, they will also likely be even more expensive. And the same will likely hold true for the myriad other potential applications of genetic engineering — they’ll be too costly to be broadly useful.
Synthego is working to dramatically reduce these costs, aiming to make gene therapies as accessible and affordable as vaccines for the people who need them.
The Human Genome, CRISPR, and the Revolution of Affordability
To understand the potential of Synthego, we need to take a few steps back to the basics of the genome and the tools we have to understand it.
Think of the genome as a very long document made up of about 3 billion pairs of 4 letters — A, G, C, and T — called “base pairs.” The letters pair in perfect predictability: an A always pairs with a T, and a C always with a G.
Base pairs group into genes; a given gene can contain thousands to millions of base pairs.
Tens to hundreds of millions of genes make up each of the 23 pairs of chromosomes that make up the nucleus of every human cell.
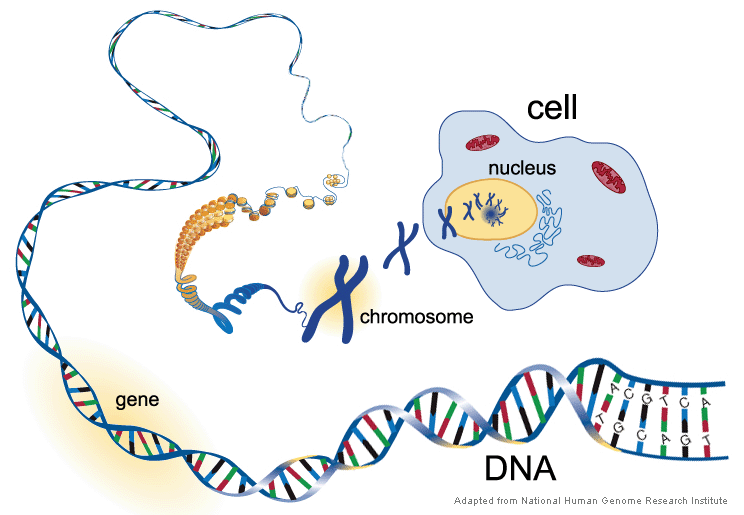
In 2001, the Human Genome Project decoded the first human genetic code revealing the order of the As, G, Cs, and Ts that made up a human in its entirety. The technical term for decoding a person’s genome is “sequencing.”
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. CRISPR were discovered in the late 1980s, and are segments of genes that contain short, repetitive sequences of base pairs. These sequences are set apart by “spacer” genes and operate as a sort of map for an enzyme called Cas9, which was co-discovered by Synthego board member Dr. Jennifer Doudna. Cas9 can be shepherded by CRISPR sequences to specific strands of letters and can be programmed to edit the letters — cutting through DNA to remove, add, or replace letters.
By modifying the sequence of letters, researchers can insert new instructions into genes and change the way cells behave, i.e. make cells produce clotting proteins where they didn’t before.
It’s like magic. But, it’s imperfect magic. Magic with errors.
The effects of such errors are not trivial. Put an A where a T is needed or a G instead of C, and the best case scenario is nothing happens. Worst case? The whole biological system is destroyed. In either case, inaccurate edits render scientific discovery unreliable — if scientists can’t be certain the edits they’re testing are the ones they meant to test, they can’t be certain of what caused the effects they observe.
The first full sequencing of the human genome in 2001 took nearly 15 years and almost $3b. As of 2016, it took a few weeks and cost as little as $1,000. The dramatic reduction in the cost of sequencing set off a revolution of microbiology, protein design, and more. It made sequencing genomes commonplace enough to produce efficiencies of scale, process improvements, and dramatically improved accuracy and reliability thanks to ever-growing data sets to analyze and compare against each other.
To make the promise of genetic engineering a reality, the same revolution for CRISPR is necessary.
Outsourcing Gene Editing for Better Outcomes
“A really interesting and important thing that we’ve identified about CRISPR,” Paul told me, “the better understanding you have and the better you can control the quality of CRISPR, the more sophisticated capabilities you have in terms of the types of edits you can do and the numbers of edits you can do simultaneously.”
Industrial control, quality, and consistency allow Synthego to thoroughly understand the complex process all the way through, in a way that would be impossible for most labs to accomplish — especially since those labs are actually focused on leveraging the process for higher order outcomes, like curing disease.
With Synthego, researchers can order CRISPR edited cells with the click of a button; the company also works with researchers to design and execute genome engineering experiments from end to end. Because Synthego has done more CRISPR edits than anyone else in the world and applies learnings from these experiments to improve its design experience and customer outcomes, it can guarantee 90% accuracy rates for cell edits.
A few of the ways Synthego is making the future a reality, today:
- Full Stack Genome Engineering: The Synthego team works with researchers through the entirety of the process, from pathway design (identifying the sequences to edit and test), through using CRISPR to make the edits, to analysis and workflow.
- Rapid Design: Scientists can design editing approaches against any of the 123,769 genomes in their design tool in less than 10 seconds. Faster yet, scientists can select and order one of several hundred cell lines with a guaranteed gene knockout. This simplifies research design and makes experimentation more consistent and reproducible.
- In-House Research: Synthego is conducting their own research in an automated and controlled environment, aiming for quality, scale, and reproducibility. As a researcher, you tell them the edit you need, and they send you the CRISPR-edited cells with a quality guarantee. The neat part is, depending on the type of experiments, they might not even have to ship cells. Instead, they can run the experiments and send you just the verified results.
- Big data: Synthego is using modeling, machine learning, and the massive amount of data it collects to better understand how the incredible number of moving parts in biology fit together. This information will allow them to do more than just efficiently and accurately produce a specific mutation in a cell. It’ll allow them to take genetic engineering a step further. Researchers will be able to specify what they want the cell to do, and Synthego will make cells to do it. At that stage, biology will have fully moved from research to synthesis. It’ll be faster, easier, and more consistent than any other technique available, and almost the whole thing will be done online.
As recently as a decade ago, computing power was localized. Every home, every company, every lab, had its own server to store and process its data. Think boxes of letters rather than inboxes of email. Plugging in external drives rather than just hitting save. “Server rooms” full of whirring computers behind the office kitchen at small businesses.
Now, companies pay for remote server farms, owned and managed by companies with specific expertise and resources to run them, and get computing power that is more powerful, reliable, and cost-effective.
Similarly, today, researchers are transitioning from shouldering the full range of costs and expertise required for CRISPR to outsourcing it to Synthego, driving quality and scale up and prices down.
“We’ll be able to do their full research workflow,” Paul explained. “If you look at how cloud computing developed, it used to be that every company handled their server farm. Now it’s all handled in the cloud. Our goal is, let’s make it as easy to do biotech research as it is to program a computer.”
Driving Humanity’s Biological Future
Through automation and high-throughput, Synthego isn’t just reducing the cost of research, it’s helping to steer it. Because biology, and especially genetics, is so complicated, a lot of current research is done through trial and error.
For example, only one out of out of every 5,000 compounds that enters drug discovery at the stage of preclinical development becomes an approved drug. This approach of trying everything to find what works is effective but inefficient.
If we could better predict, at a genetic and molecular level, what a drug or treatment would do, we could flip this model on its head. We’ll be able to design drugs and treatments based on how we believe they’ll function based on precise, well-understood models.
For gene therapies, this requires an understanding not only of how the therapy will work but also of the best way to deliver it. Synthego’s work will help with both.
In the not so distant future, gene therapies may be similar to vaccines. With genetic sequencing prices continuing to drop, and our understanding of what causes various diseases at the genetic level continuing to improve, individuals may be able to vaccinate themselves before the symptoms of a disease even emerge.
People with harmful mutations in their BRCA1 or BRCA2 genes are at much higher risk for ovarian and breast cancers. With gene therapies, these people could opt to be genetically vaccinated, repairing the mutations, before cancer can develop.
Huntington’s disease is a genetic condition caused by extra repeats of a three letter sequence, “CAG,” in the Huntington Gene, a normal part of the fourth chromosome. The disease causes progressive brain cell death, starting between ages 30 and 50. There is no cure, and even the best treatments only moderately improve quality of life. With gene therapies, we could remove the extra copies of the “CAG” triplet from the DNA of at-risk individuals, preventing the disease from ever developing.
Just like the vaccines we have today for certain viral and bacterial diseases, genetic vaccines should accessible to everyone. For the first time in human history, we have a chance to control our own biological future, but if we’re going to create a future that is equitable and healthy, accessibility needs to be a top priority.
I’m glad Synthego is on the case.
Share
Return to News